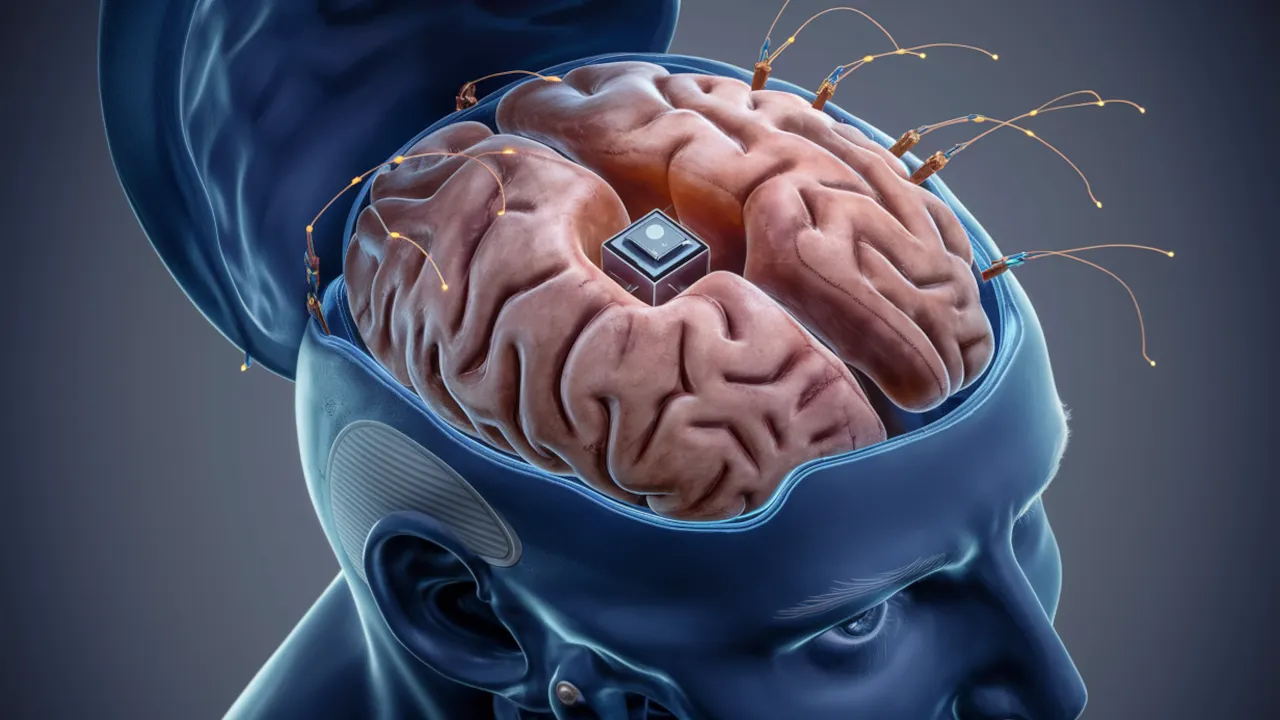
Elon Musk, CEO of Tesla, SpaceX, and Neuralink, announced today via a tweet that Neuralink is looking for its second participant to undergo a brain implant trial. In true Musk fashion, the tweet described the implant as a "telepathy cybernetic brain implant that allows you to control your phone and computer just by thinking."
Neuralink is a company developed by Musk that aims to build brain-computer interfaces (BCIs). The goal of these BCIs is to enable individuals with conditions such as paralysis to control digital devices using their thoughts alone.
Musk did not provide any additional details regarding the trial—nor did he clarify whether he was being serious or not with his description—and Neuralink has not posted any information about it on its social media platforms. However, the company's latest PRIME study update invites people to apply as test subjects for its clinical trial.
PRIME, which stands for "Pilot Research Implant for Medical Experimentation," is Neuralink's clinical trial that aims to evaluate the safety and initial functionality of its fully implantable, wireless BCI device, the N1 chip. The chip is highly invasive, involving thousands of tiny threads penetrating the brain.
Neuralink is accepting applications for the second participant.
This is our Telepathy cybernetic brain implant that allows you to control your phone and computer just by thinking.
No one better than Noland (@ModdedQuad) himself to tell you about the first! https://t.co/k9DaZ3xr5g
— Elon Musk (@elonmusk) May 17, 2024
Neuralink has faced controversy in the past, with experts assuring that its animal trials resulted in the deaths of over a thousand animals—which Musk denies—and that Neuralink failed to provide any documentation related to the calibration and maintenance of its “vital signs monitor.”
In January, Neuralink implanted its device in the brain of its first patient, Noland Arbaugh. Arbaugh, who is paralyzed from the shoulders down due to a diving accident in 2016, says that he has experienced significant progress with the device.
He can now play video games, browse the internet, and control a computer cursor on his laptop using only his thoughts. According to Neuralink, Arbaugh quickly surpassed the world record for cursor control speed after the surgery.
Last week, Neuralink revealed that the implant's tiny wires inside the brain of its first patient moved out of position during the first human trial, resulting in fewer electrodes to measure brain signals. The company managed to restore the implant's function by making adjustments, including refining its algorithm to enhance sensitivity.
To counter these risks, new alternatives are popping up from rival companies—like Precision Neuroscience, which opts for what it claims is a safer, less invasive approach by coating the brain with a sort of electrical blanket to register its activity.
The eligibility criteria for the Neuralink trial include individuals within the United States or Canada, aged 18 or older, who have quadriplegia, paraplegia, vision loss, hearing loss, the inability to speak, and/or major limb amputation (above or below the elbow and/or knee), and are able to consent. Prospective participants can apply by visiting Neuralink's Patient Registry.
Edited by Andrew Hayward